Discover our portfolio of innovative diagnostic solutions
SkylineDx engages with (academic) research institutes and technology partners to develop precision diagnostics. Before a new molecular biomarker enters our Research & Development Programs, it is thoroughly evaluated in a diligent process captured by our innovation engine STRIX. STRIX ensures that a potential new biomarker is not just academically promising but also clinically relevant.
Within our R&D Programs, personified by animal genera, the biomarker is optimized and its performance verified. A controlled design and development process follows in which both the analytical and the clinical validation are tested. When the biomarker, device, and generated data pass regulatory requirements, it is branded and launched as a commercial test to serve a clinical solution.
Research & Development
Our teams are structured to advance the research and development in 3 dedicated disease programs: dermatology, hematology, and inflammatory diseases.

Before a new molecular biomarker enters our Research & Development Programs, all discoveries and research opportunities are thoroughly evaluated in our dedicated STRIX (Systematic, Translational R&D Workflow, building on Integrated Expertise) Program. Our interdisciplinary team searches, screens, and evaluates hundreds of high-potential biomarkers a year, selecting the most promising. We then partner with the inventor and potentially license the related intellectual property (IP) that allows us to develop and commercialize the biomarker.
We form strong partnerships with technology transfer offices from hospitals and academia, as well as close collaboration with the involved clinicians and researchers directly. When a biomarker progresses from STRIX to one of our R&D Programs, we might file and develop additional IP.
Strix is a genus of the owl family and symbolizes wisdom and perception. Its sharp perception of details, broad view, and ability to see in the dark closely resembles what the STRIX Program is capable of making visible which would otherwise remain hidden.
Do you want to discuss opportunities for partnership?
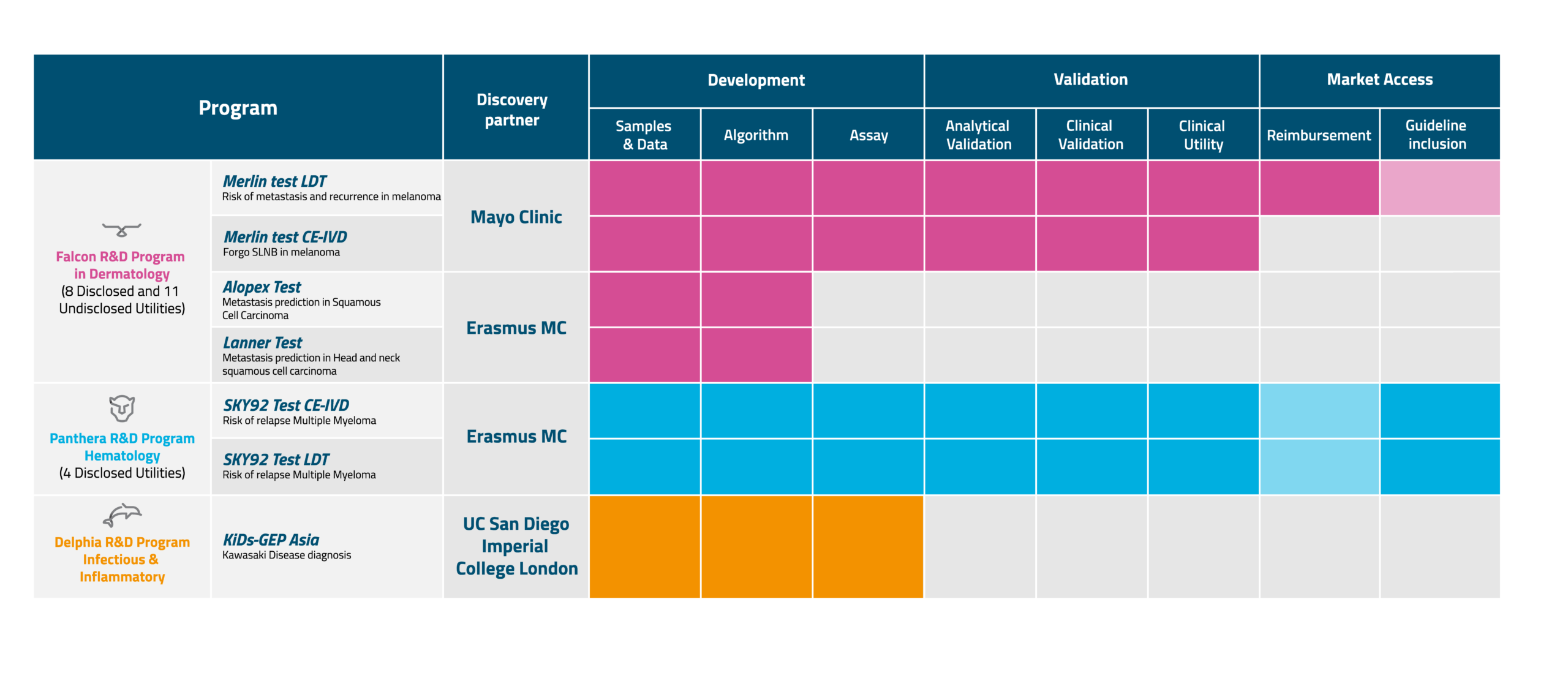
The American Association of Dermatology estimates that 1 out of 5 Americans will develop skin cancer by the age of 70. When detected early, the 5-year survival rate for melanoma is 99%. Unfortunately, misdiagnoses often occur due to the similarities between skin lesions. Our Falcon R&D Program is uniquely equipped to unveil new, detailed insights into the genomic, biological, and clinical nature of melanoma, squamous cell carcinoma, and other aspects of dermato-oncology (skin cancer). Under the wings of the Falcon R&D Program, a series of specific studies and projects have been initiated, aimed at developing and introducing an array of diagnostic utilities, to support decision-making by care providers and patients at multiple points on their treatment journey.
The Merlin™ test is the first precision diagnostic assay from this program that has been commercially launched in Europe and the United States. More specifically, this test determines if a patient is at low risk for having metastasis in their sentinel lymph node. These patients could potentially forgo invasive surgery under general anesthetics and are not subjected to the related complications including hospital readmissions.
A merlin is a small, fierce species of Falcon that can powerfully hone in on its prey, just like our melanoma test kit. For more information, please scroll down to our clinical solutions.


The Panthera Program focuses on hematological malignancies, cancers that develop in blood cells. Tackling hematological cancer requires strength and intelligence. Conditions such as multiple myeloma hide deep in the bone marrow. Panthers are controlled hunters who can detect their prey between dense thickets, with their keen senses. They have patience and are accurate on a detailed level as they rule their terrain.
SKY92 is the first precision diagnostic assay from this program that has been commercially launched in Europe and the United States. More specifically, this test provides care providers and patients insights into how aggressive the myeloma is, by looking at 92 genes in the malignant plasma cells. In general, a more aggressive myeloma, called high-risk myeloma, requires an intensified treatment strategy. For more information, please scroll down to our clinical solutions.
Infectious and Inflammatory diseases consist of many disorders and studies suggest that a dysfunction of the immune system seems to be an important shared characteristic. Well-known diseases include AIDS, inflammatory bowel disease (IBD), Type 1 Diabetes, asthma and COVID.
Much less known is Kawasaki disease. A systemic vasculitis (inflammation of the blood vessels) that most commonly occurs in children under 5. The disease may result in the development of coronary artery aneurysms (bulge or ballooning in blood vessels of the heart), which may cause cardiac problems. Diagnosing Kawasaki disease is challenging since symptoms cannot always be recognized. Effective treatment is available, but having a timely diagnosis is crucial. In collaboration with Imperial College London and the University of California San Diego School of Medicine, we are developing a diagnostic test that will facilitate early diagnosis of Kawasaki Disease.
Delphinidae is the family name of 36 species of dolphins, living in nearly all aquatic environments These mammals understand inflammation through movement where they swim fairly slow as the body has it under control and reach 50 km/h as the disease flames up. To combat the (auto)immune response, dolphins are one of the few species that have the ability to recognize themselves in the mirror. Tackling these types of diseases requires the dolphin’s reflective intelligence and effective use of tools.

Genomics are playing an increasingly important role in offering a personalized treatment path to patients and thereby improving their quality of life. SkylineDx bridges the gap between academically discovered gene-based biomarkers and commercially available diagnostic products. Whether it is oncology or another disease area, we continuously strive to offer the best diagnostic solutions that address a clinical unmet need and ultimately support care providers and patients in their treatment decision-making.

The Merlin™ test is our first clinically available test resulting from Falcon R&D Program. The test has been developed in partnership with Mayo Clinic (United States) and is validated thoroughly in large multicenter trials in the United States, Europe, and Australia.
The Merlin™ test uses the CP-GEP algorithm. A powerful model that calculates the patient’s risk of having metastasis in their sentinel lymph nodes. CP-GEP calculates this risk on an individual basis by analyzing 8 genes from the patient’s primary tumor, the tumor thickness, and the patient’s age.
How does the Merlin™ test support care providers and patients in their decision-making? The current standard to determine if a patient has metastasis in their sentinel lymph nodes, is an invasive surgery under general anesthetics, called a sentinel lymph node biopsy. In about 80% of patients, no metastasis is found. For these patients, the surgery has no discernable therapeutic effect but the patient will have a >10% risk of surgery-related complications, sometimes severe enough for a hospital readmission. If the Merlin™ test is applied before the surgery and provides a high-risk result, the patient will most likely still be referred for the surgery. However, when Merlin provides a low-risk result, the patient may forgo the surgery, leading to a reduction of many surgeries and surgery-related complications.
SKY92 is our first clinically available test resulting from Panthera R&D Program. The test has been developed in partnership with Erasmus MC (Rotterdam, the Netherlands) and is the most validated test on the market to determine a multiple myeloma patient’s prognosis.
SKY92 is a very robust model that analyzes the activity of 92 genes in the malignant plasma cells. How does SKY92 support care providers and patients in their decision-making? Multiple myeloma is a relapsing-remitting disease. This means that therapy might keep the disease under control for a period of time (remission) but eventually, the patient experiences a relapse after which the disease usually progresses. Patients with high-risk myeloma, have a more aggressive disease and experience shorter remission periods and fast relapses. Clinical guidelines recommend high-risk patients to be treated more intensively in an attempt to keep the patient in remission longer. SKY92 is such a high-risk myeloma test. If SKY92 is applied at diagnosis, it will classify the patient as high risk or not. In case of a high-risk result, the patient and care provider might decide to follow an intensified treatment regimen.


SkylineDx is ISO 13485:2016 certified