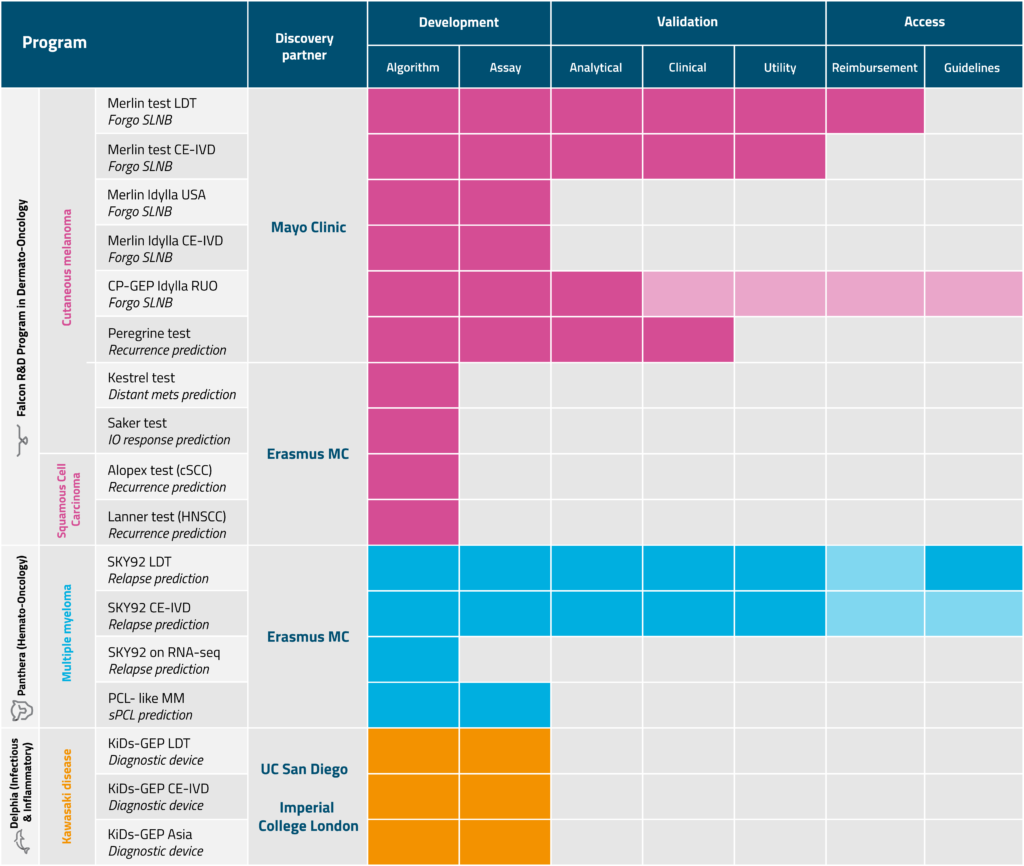
Transforming diagnosis and treatment planning through advanced gene expression profiling
Multiple Myeloma (MM) is a complex and genetically diverse blood cancer that originates in plasma cells, specialized white blood cells found in the bone marrow. It is the second most common blood cancer.
MM develops when malignant plasma cells multiply uncontrollably in the bone marrow, leading to an excessive buildup of abnormal myeloma cells. These cells produce monoclonal proteins (M-proteins), which can cause serious complications, including:
MM is a highly heterogeneous disease, meaning it varies significantly from patient to patient in terms of genetic profile, disease progression, and treatment response.
MM typically follows a relapsing-remitting pattern, where treatment becomes less effective with each recurrence. This makes accurate risk assessment at diagnosis critical for selecting the right treatment approach especially during first-line therapy, which has a major impact on long-term outcomes.
While MM remains a serious and currently incurable condition, advances in molecular diagnostics and targeted therapies are improving the ability to predict disease behaviour and personalize care, offering new hope for better outcomes.
SKY92 is a molecular diagnostic test developed by SkylineDx designed specifically for patients with Multiple Myeloma (MM) — both newly diagnosed and those with relapsed or refractory disease.
It analyses the expression of 92 genes — known as the SKY92 signature — in myeloma cells to uncover prognostic information not available through standard diagnostic methods.
SKY92 empowers clinicians with more precise risk stratification, enabling informed treatment decisions which can improve the outcomes of patients.
The SKY92 test provides two possible outcomes:
High Risk
indicates a higher likelihood of aggressive, progressive disease and reduced overall survival.
Standard Risk
indicates a more favourable prognosis, with a lower risk of rapid disease progression and better expected survival outcomes.

Bone marrow aspirate collected from the patient
CD138+ plasma cells isolated within 32 hours
RNA extracted and analysed for expression of 92 genes
Data processed through a validated algorithm
Patient is stratified into standard-risk or high-risk
Multiple Myeloma (MM) is a highly heterogeneous disease, meaning it does not behave the same in every patient. While some individuals experience a slow-growing, manageable form, others develop aggressive disease that relapses quickly, even after initial treatment success.
Approximately 20–25% of newly diagnosed patients fall into this high-risk category, often driven by underlying genetic abnormalities in their myeloma cells.
MM typically follows a relapsing-remitting pattern, where treatment becomes less effective with each recurrence. This makes the first line of therapy especially critical, as a strong initial response can significantly impact long-term outcomes.
Identifying a patient’s risk level at diagnosis allows doctors to tailor treatment strategies more effectively:
Despite the importance of early risk assessment, many of the tools used in routine practice lack standardization, leading to inconsistent or unclear results. As a result, patients may miss key opportunities, such as enrolling in clinical trials suited to their risk level, receiving the right intensity of treatment (either more aggressive or less intensive), or accessing timely supportive care when they need it most.
MM typically follows a relapsing-remitting pattern, where treatment becomes less effective with each recurrence. This makes accurate risk assessment at diagnosis critical for selecting the right treatment approach especially during first-line therapy, which has a major impact on long-term outcomes.
While MM remains a serious and currently incurable condition, advances in molecular diagnostics and targeted therapies are improving the ability to predict disease behaviour and personalize care, offering new hope for better outcomes.
SKY92 provides a standardized, risk classification based on tumour biology/genomics during diagnosis, helping clinicians make confident, personalized treatment decisions. By revealing the disease’s biological behaviour early, it guides clinicians providing better targeted strategies, potentially improving outcomes for both high- and standard-risk patients.
Traditional clinical markers like FISH often miss a subset of aggressive myeloma cases. The SKY92 gene expression profile identifies 10–15% of high-risk patients who are not detected by standard tests, enabling earlier, more tailored intervention. This finding has been consistently validated in multiple clinical trials worldwide.
Discovery SKY92 signature (Kuiper et al.)
A gene expression signature for high-risk multiple myeloma | Leukemia
CE-IVD test certification
ProMMis publication
https://onlinelibrary.wiley.com/doi/abs/10.1111/bjh.20050
Contact Us to Order the Test
While traditional tests like FISH (Fluorescence In Situ Hybridization) identify certain chromosomal abnormalities, MMprofiler detects gene expression patterns that reveal biological behavior missed by standard methods. In fact, MMprofiler identifies 10–15% of high-risk patients not captured by clinical markers like FISH.
MMprofiler is suitable for patients with newly diagnosed or relapsed/refractory Multiple Myeloma, especially when a more precise risk assessment could impact treatment planning. It is typically ordered by hematologists or oncologists managing MM care.
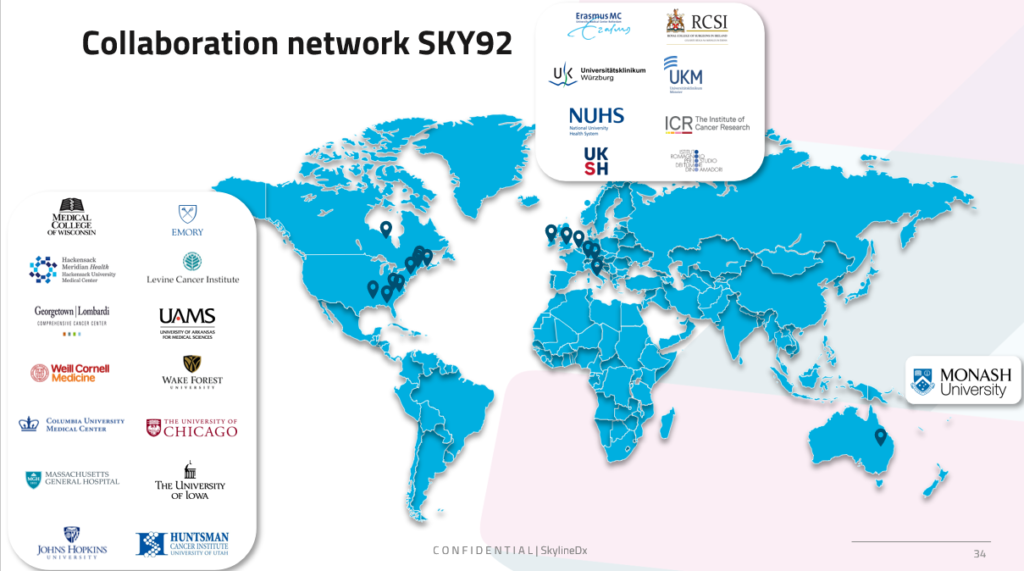
MMprofiler has been analytically and clinically validated in multiple international studies.
MMprofiler supports personalized medicine by:
The MMprofiler test is completed in approximately 4 working days in the laboratory. In a clinical setting, accounting for sample logistics and processing workflows, results are typically delivered in less than 3 weeks from the time of sample collection (Ref)
Unfortunately, MMprofiler is not available globally, if not available in your country please make an inquiry via (EMAIL)
The tests have been clinically validated by X international institutions, to know more, read our publications
ROTTERDAM (the Netherlands), SAN DIEGO (CA, USA), January 8th, 2025— SkylineDx, an innovative diagnostics company specializing in the research and development of molecular diagnostics for dermatology, hematology, and infectious & inflammatory diseases,...
Rotterdam, The Netherlands – San Diego, CA – [December 18, 2025] –— SkylineDx today announced the release of new data from two complementary, peer-reviewed publications that reinforce the clinical value of the...
ROTTERDAM, The Netherlands and SAN DIEGO, November 6, 2025 – SkylineDx, an innovative diagnostics company specializing in the research and development of molecular diagnostics for oncology, and inflammatory and infectious diseases, today announced...
Rotterdam, The Netherlands – San Diego, CA – [October 22nd] – SkylineDx today announced that results from its landmark MERLIN_001 trial, the largest prospective evaluation of a genomic test in cutaneous melanoma,...
ROTTERDAM (the Netherlands), SAN DIEGO (CA, USA), September 9th, 2025— SkylineDx, an innovative diagnostics company specializing in the research and development of molecular diagnostics for oncology, and inflammatory and infectious diseases, will...
ROTTERDAM (the Netherlands), SAN DIEGO (CA, USA), July 22nd, 2025— SkylineDx, an innovative diagnostics company specializing in the research and development of molecular diagnostics for oncology, inflammatory and infectious diseases, today announced...